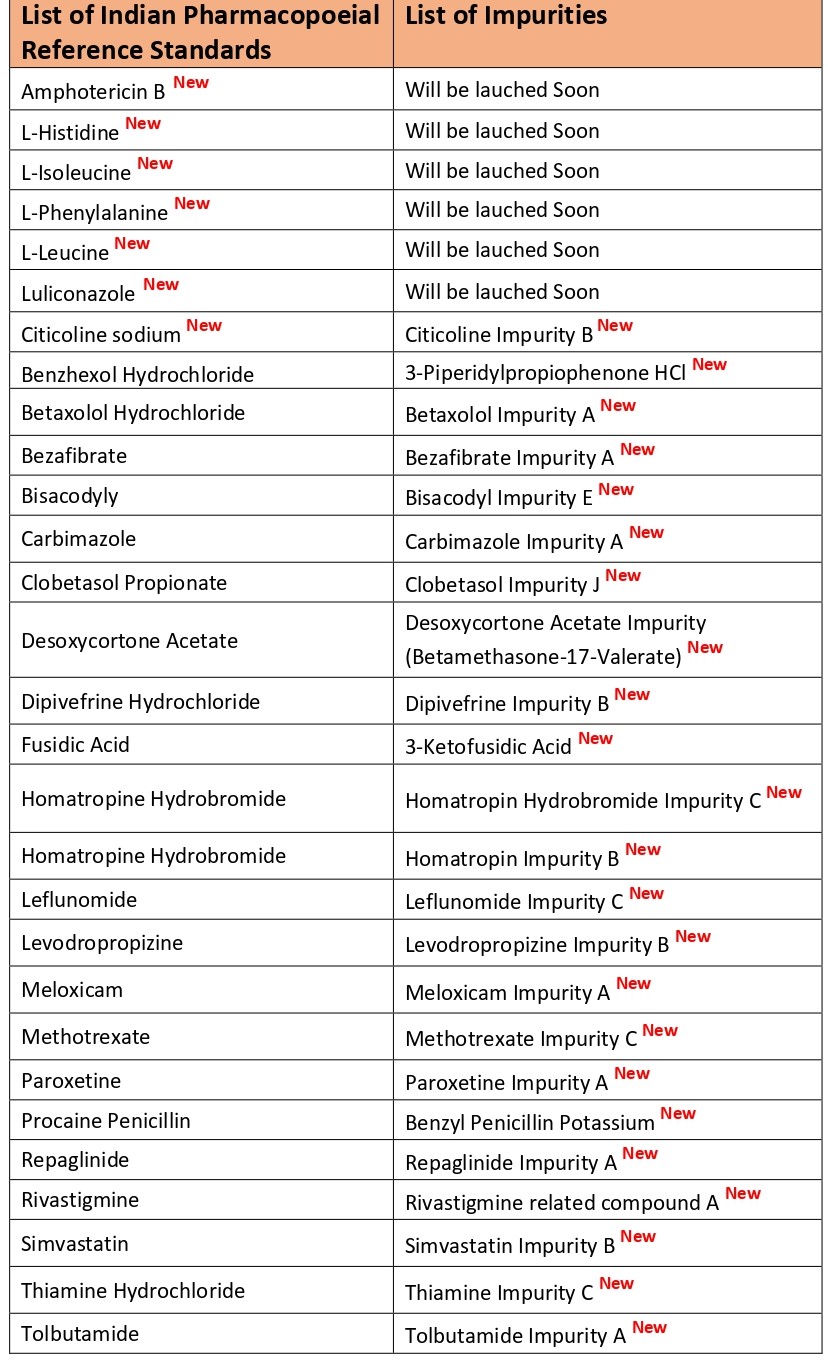
The Indian Pharmacopoeia Commission (IPC) has added 23 new impurities standards and 7 new Indian Pharmacopoeial Reference standards

he IPC in a release has announced a list of 23 new impurities standards and seven new Indian Pharmacopoeial reference standards.
Impurity standards are used to perform the system suitability, qualitative and quantitative parameters for compliance to Indian Pharmacopoeia monograph. The IPC also added that a few monographs require the use of a chemical reference substance or a biological reference preparation.
These are authentic specimens chosen and verified based on their suitability for intended use as prescribed in the pharmacopoeia and are not necessarily suitable in other circumstances.
IP reference substances, abbreviated to IPRS are the official standards issued by the IPC. They are the official standards to be used in cases of arbitration.
The vision of IPC is to promote the highest standards of drugs for use in humans and animals within practical limits of the technologies available for manufacture and analysis.
The mandate of the IPC is to develop comprehensive monographs for drugs to be included in the Indian Pharmacopoeia, including active pharmaceutical ingredients, pharmaceutical aids and dosage forms as well as medical devices and to keep them updated by revision regularly. To develop monographs for herbal drugs, both raw drugs and extracts/formulations therefrom. To accord priority to monographs of drugs included in the National Essential Medicines List and their dosage forms.
To review existing monographs periodically to delete obsolete ones and amend those requiring upgrading /revision.
To organize educational programs and research activities for spreading and establishing awareness on the need and scope of quality standards for drugs and related articles /materials.
To publish the National Formulary of India for updating medical practitioners and other healthcare professionals.
To act as a National Coordination Centre for Pharmacovigilance Programme of India.

Subscribe To Our Newsletter & Stay Updated