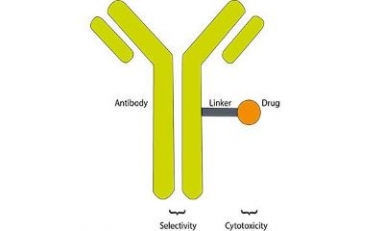
Lonza acquires biotech firm Synaffix
Acquisition will further strengthen Lonza’s bioconjugates offering through the integration of the industry-leading proprietary Synaffix technology platform and R&D capabilities, including payload and site-specific linker technology