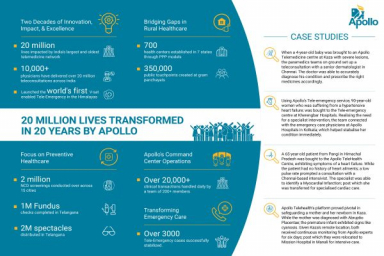
Solvay launches new PVDC coating solution for more sustainable pharma blister films
Diofan Ultra736 provides ultra-high water vapor barrier with excellent thermoformability, unlocking the potential to design thinner and more sustainable blister films