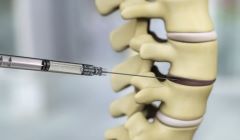
DiscGenics presents positive Phase1/2 study of Cell therapy for degenerative disc disease
Allogeneic discogenic cell therapy was well tolerated and produced clinically meaningful, statistically significant improvements in low back pain, function, and quality of life by 12 weeks following intradiscal injection