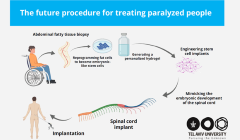
CUHK researchers discover distinct gut microbial signatures for prediction, diagnosis and treatment of long Covid
These distinct gut microbial signatures can be used to predict the risk of developing long Covid and diagnose long Covid in patients with persistent symptoms after the acute infection