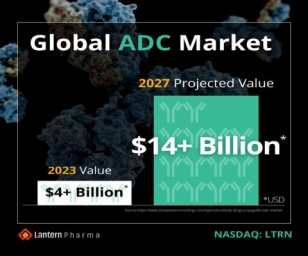
Lantern Pharma leverages AI platform to develop breakthrough ADCs with Bielefeld University
Lantern is receiving an exclusive and worldwide option to license intellectual property from Bielefeld University related to the collaboration and IP generated from the collaboration