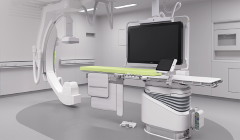
Philips instals 1000 active cath labs in India
Over the next few years, Philips aims to double the number of its active cath labs in India with a special focus on improving accessibility to quality cardiac and neurovascular care in tier 2 and tier 3 cities



_256_384.jpg)








