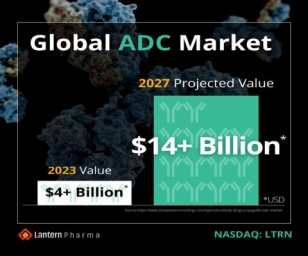
Seven manufacturers sign sublicence agreements with the MPP to produce generic versions of Shionogi's COVID-19 oral antiviral ensitrelvir
Ensitrelvir is an oral antiviral that suppresses the replication of SARS-CoV-2 by selectively inhibiting the viral 3CL protease.