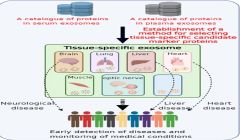
NIBIOHN identifies tissue-specific exosome marker candidates from blood
Blood contains a mixture of exosomes secreted from nearly all tissues and cells, and exosomes from other tissues can mask changes caused by disease, making it difficult to detect the presence of biomarkers that reflect disease