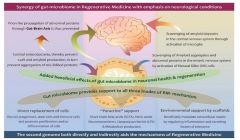
Sumitomo Pharma Oncology receives orphan drug designation for the treatment of myelofibrosis
TP-3654 is currently being evaluated in a Phase 1/2, multicenter, dose-escalation, open-label trial to assess safety, tolerability, pharmacokinetics, and pharmacodynamics in patients with intermediate or high-risk primary or secondary myelofibrosis.