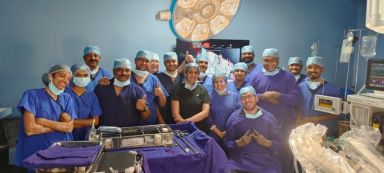
Kokilaben Dhirubhai Ambani Hospital makes history with India’s first intercontinental remote robotic surgeries
The Toumai platform, currently the only robotic system with US FDA Study Approval for telesurgery, enabled real-time surgical control with no compromise to patient safety