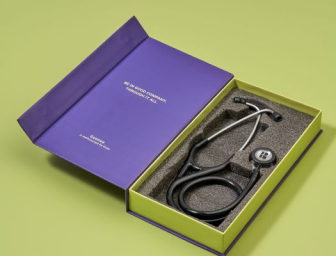
Knya forays into medical equipment category with '6sense Stethoscope'
The stethoscope features high acoustic sensitivity and active noise cancellation, minimizing ambient interference to improve auscultation clarity, particularly in noisy hospital settings