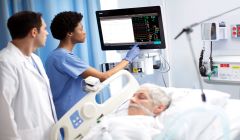
Philips receives FDA 510(k) clearance for acute patient monitors
Philips Patient Monitors IntelliVue MX750 and IntelliVue MX850 pair with advanced software and services to offer clinical decision support, continuous, scalable patient management and enhanced infection control