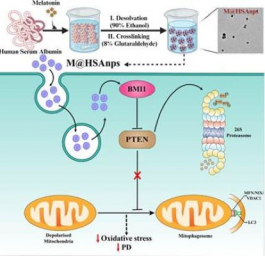
Merck unveils formulation and technology center in Navi Mumbai
The Formulation & Technology Centre intends to function as a technology storefront, offering advanced formulation and processing solutions to address the growing demand for complex drug formulations