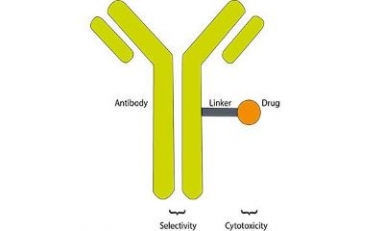
CHMP adopts positive opinion recommending approval of Bristol Myers Squibb’s Opdivo in combination with Cisplatin and Gemcitabine
If approved, the Opdivo-based regimen would be the first immunotherapy-chemotherapy combination approved for this patient population in the EU