The companies expect to manufacture over 80 million does of the Covid-19 vaccine in 2022
Expanding access to its Plasmid DNA based Covid-19 (ZyCoV-D), vaccine, Cadila Healthcare has entered into a manufacturing license and technology transfer agreement with Enzychen Lifesciences of South Korea.
Both companies believe that this partnership will lead to the estimated manufacturing of 80 million or more doses of the Plasmid DNA vaccine in 2022. Cadila will provide a manufacturing licence and transfer of the technology to Enzychen.
Under the agreement, Enzychem will manufacture and commercialise the vaccine within its territory under a Zydus trademark and Cadila will receive the license fees and royalty payments for the same.
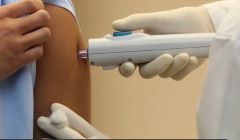
ZyCoV-D is the first DNA plasmid vaccine in the world for human use, developed indigenously by Cadila against the Covid-19 virus. The vaccine is administered intradermally using the PharmaJet needle-free applicator, which ensures painless intradermal vaccine delivery.
The three-dose vaccine is administered 28 days apart from one another.
Speaking on the development, Dr. Sharvil Patel, Managing Director, Cadila Healthcare Ltd., said "We are very happy to partner with Enzychem Lifesciences and provide access to the technology behind the needle-free, Plasmid DNA vaccine technology which is the first of its kind in the world. Our aim is to provide new innovations and novel technologies that can support people with better approaches to healthcare. This agreement enables people in South Korea and other key markets of Enzychem, the access to a safe, well tolerated and efficacious vaccine with a novel platform to fight COVID-19."
Ki Young Sohn, Chairman of Enzychem Lifesciences said: "Today marks an important milestone for our vaccine consortium as we embark on the manufacturing of the world's first in-class DNA vaccine for humans. This technology allows vaccines to be produced at affordable costs in record times, and DNA vaccines are considerably stable. ZyCoV-D is administered without needles and can be deployed more readily, especially in resource-poor populations where these are urgently needed". "With state-of-the-art manufacturing facilities and a qualified workforce dedicated to manufacturing high quality products, we are very eager to partner with Zydus in addressing the global demand for Covid-19 vaccines, especially in low-medium income countries."

Subscribe To Our Newsletter & Stay Updated