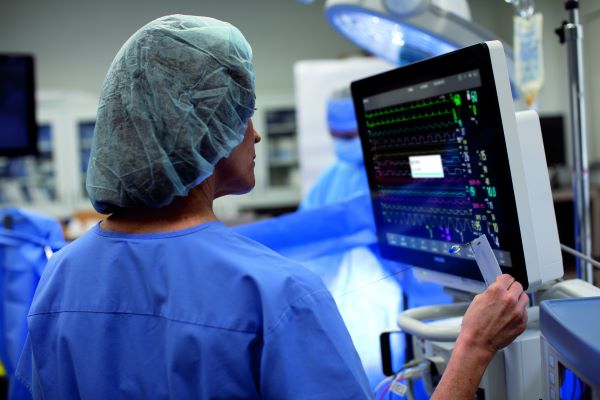
Philips and Masimo introduce advanced monitoring capabilities to Philips high acuity patient monitors
The latest extension of Masimo and Philips’ ongoing collaboration will help enable clinicians to make quick and informed decisions without the need for additional monitoring equipment





.jpg)