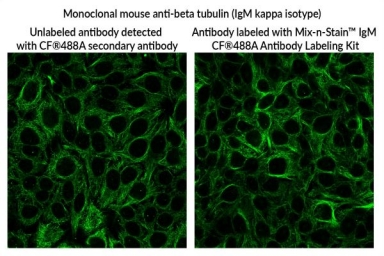
Biotium introduces novel antibody labeling Kits for efficient labeling of IgM antibodies
Mix-n-Stain CF Dye IgM Antibody Labeling Kits address longstanding challenges for labeling IgM antibodies by offering efficient and convenient conjugation with bright CF Dyes