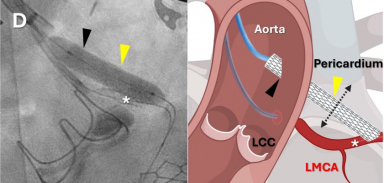
Bayer acquires Attralus’ cardiac amyloidosis imaging agents
The deal to acquire AT-01 (124-Iodine-evuzamitide) and AT-05 (PAR-Peptide + technetium-99m) underscores Bayer’s commitment to expanding in molecular imaging and advancing precision cardiology