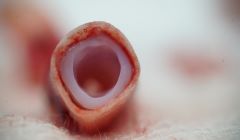
World’s first clinical trial for 3D bioprinted stem cell vascular grafts in China
Revotek announced that it has received clearance from the National Health Commission of China to begin a clinical study in the West China Hospital, Chengdu, with its first stem cell 3D bioprinting product, REVOVAS.