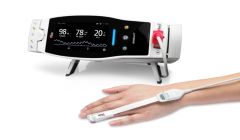
New study finds that Masimo PVi may be useful in helping ER doctors determine the severity of asthma attacks
Acute asthma attack is a common cause of admission to emergency departments (EDs) among children, and triage by severity is important for determining appropriate clinical treatment