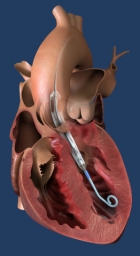
Second-Gen mRNA COVID-19 vaccine candidate CV2CoV show positive signs in preclinical study
The preclinical study provides evidence for strongly improved immune responses with second-generation mRNA backbone jointly developed by CureVac and GSK compared to CureVac’s first-generation mRNA backbone