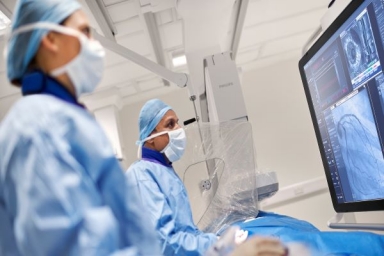
Philips’ image-guided navigation increases safety during coronary interventions
Late-breaking data from DCR4Contrast study presented at EuroPCR 2023 and SCAI 2023 also shows an average reduction in the total number of angiograms obtained during each procedure of 26.3%