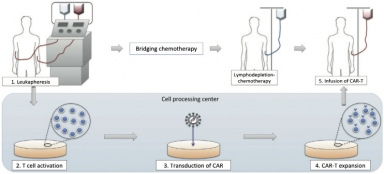
Growing investment in biotech startups likely to make CAR-T therapies affordable in India, says GlobalData
GlobalData’s ‘Pharmaceutical Intelligence Center’ observes that India is still in the nascent stage of developing these potential therapies as currently only four cell/gene therapy molecules are being developed in the country.