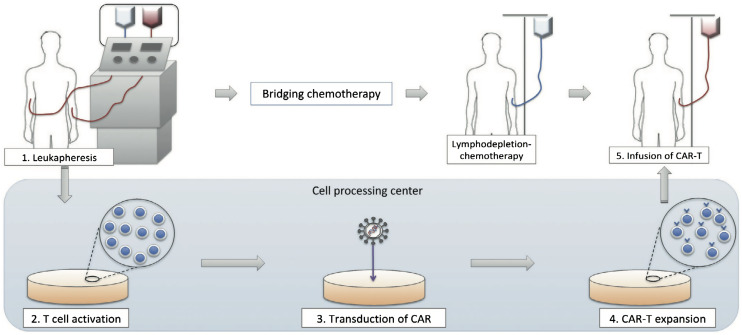
Biotech startups in India, which are focused on developing cell/gene (including CAR-T) therapies for the management and treatment of certain life-limiting illnesses that significantly affect the patient’s quality of life, are increasingly attracting investment from major pharmaceutical companies. This is likely to boost their research and production capabilities and eventually makes the therapies affordable to those in need, says GlobalData.
GlobalData’s ‘Pharmaceutical Intelligence Center’ observes that India is still in the nascent stage of developing these potential therapies as currently only four cell/gene therapy molecules are being developed in the country.
Immuneel Therapeutics’ IMN-003A (Phase II) and ImmunoACT’s gene therapy HCAR-19 (Phase I) are the CAR-T therapies being studied for hematological cancers. Stempeutics Research’s Stempeucel is a cell therapy in Phase III development for osteoarthritis and diabetic foot ulcers, and in Phase II for Crohn’s disease. Cancer Institute WIA’s cellular immunotherapy is being studied in Phase II for HPV-associated cervical cancer and in Phase I for ovarian cancer.
Neha Myneni, Pharma Analyst at GlobalData, comments: “Though CAR-T cell therapies have already shown promising results in blood cancer patients who have exhausted all other means of treatment, they come with a high price tag, making them difficult to access. Biotech startups in India are working towards making these expensive therapies affordable.”
According to GlobalData’s ‘Pharmaceutical Intelligence Center’, the following Indian biotech startups involved in the development of cell and gene therapies have received investments in the past year:
India has a growing cancer burden as the country is expected to see a 12% rise in cancer cases during the next five years to 29.8 million, according to the Indian Council of Medical Research.
Myneni concludes: “Due to the lack of availability of CAR-T cell therapies in India, patients need to travel to other countries to avail the treatment. The average CAR-T therapy treatment cost in the US is approximately $400,000 – $500,000. Through strategic partnerships and enhanced funding opportunities, the budding biotech startups in India are focusing on strengthening their in-house R&D capabilities for these potential therapies.
“As Laurus Labs backed startup ImmunoACT plans to make HCAR-19 available in India through various cancer hospitals across the country from 2023, this brings a ray of hope for blood cancer and lymphoma patients to avail these therapies at much more affordable cost i.e., INR 2-3 million (approximately $25,000 – $38,000). The success of these pipeline candidates and their eventual affordability to patients is likely to have a significant impact on the treatment of these incurable diseases both in India as well as on a global scale.”