Edwards’ latest-generation balloon-expandable transcatheter heart valve incorporates a proprietary calcification-resistant tissue technology
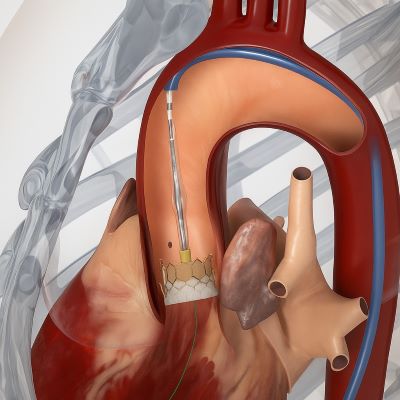
Edwards Lifesciences, a global leader in structural heart innovations, has announced the launch of its next-generation transcatheter heart valve platform in India, marking a significant milestone in the treatment of aortic stenosis.
This advanced therapy is designed to meet the needs of patients who are not candidates for open-heart surgery, offering a durable, minimally invasive solution that supports long-term health and quality of life.
Aortic stenosis is a progressive, degenerative condition often linked to aging, caused by calcification of the aortic valve that restricts blood flow from the heart. Once symptoms appear, nearly 50% of patients face mortality within two years without timely intervention, underscoring the urgency of early diagnosis and treatment. According to the Global Burden of Disease study, in India, a large number of deaths are attributed to severe calcific non-rheumatic aortic stenosis.
Edwards’ latest-generation balloon-expandable transcatheter heart valve incorporates a proprietary calcification-resistant tissue technology that helps prevent calcium buildup on the valve leaflets, which is a primary cause of structural valve deterioration and the need for reintervention. The valve also features dry tissue storage, simplifying hospital workflows, and a taller, textured outer sealing skirt to reduce paravalvular leak.
Commenting on the Edwards’ next-generation transcatheter heart valve platform, reputed cardiologists from India shared their perspectives. Dr. Ravinder Singh Rao, Interventional Cardiologist, Lilavati Hospital Mumbai said, “The newer transcatheter heart valve, which is more durable and long-lasting, will allow us to safely and effectively treat younger patients with severe aortic stenosis.”
“Durability is the key for evaluating any transcatheter heart valve. With a strong clinical record of proving to be the most durable valve in the market, the patients will benefit from long-term disease management with this launch,” remarked Dr. G. Sengottuvelu, Interventional Cardiologist, Apollo Hospital, Chennai.
Dr. A B Gopalamurugan, Interventional Cardiologist, MGM Healthcare, Chennai said, “We have waited a long time for this technology to reach India. With this launch, patients here will now have access to the best-in-class transcatheter heart valve technology, within the comfort of their own country.”
Parameswaran Nair, Country Leader of India and Southeast Asia, Edwards Lifesciences, commented: “At Edwards Lifesciences, we are deeply committed to pioneering innovations that redefine care for structural heart disease. By integrating minimally invasive techniques with exceptional durability, we are delivering therapies that meaningfully enhance survival and quality of life for patients with aortic stenosis in India. This milestone reflects our dedication to empowering clinicians, expanding access to care, and addressing the distinct needs of younger patients requiring lifelong treatment strategies.”

Subscribe To Our Newsletter & Stay Updated